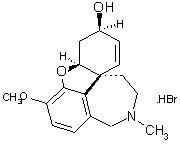
PRdomain.com | Ranbaxy Laboratories| Ranbaxy to manufacture and market galantamine tablets Ranbaxy Laboratories Limited (RLL), announced today that the Company has received tentative approval from the U.S. Food and Drug Administration to manufacture and market Galantamine Hydrobromide Tablets, 4mg (base), 8mg (base), and 12mg (base). Galantamine is indicated for the treatment of mild to moderate dementia of the Alzheimer’s type. Total annual market sales for Razadyne* were $130.0 million (IMS – MAT: June 2007).Ranbaxy Laboratories Limited, India's largest pharmaceutical company, headquartered in India, is an integrated, research based, international pharmaceutical company producing a wide range of quality, affordable generic medicines, trusted by healthcare professionals and patients across geographies. Ranbaxy’s continued focus on R D has resulted in several approvals in developed markets and significant progress in New Drug Discovery Research. The Company’s foray into Novel Drug Delivery Systems has led to proprietary "platform technologies,” resulting in a number of products under development. The Company is serving its customers in over 125 countries and has an expanding international portfolio of affiliates, joint ventures and alliances, ground operations in 49 countries and manufacturing operations in 11 countries.