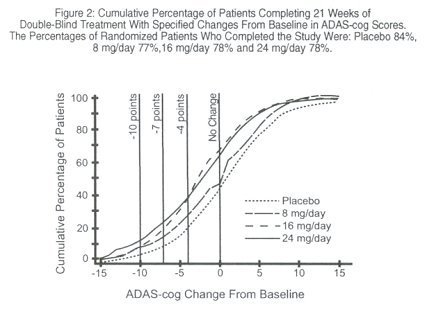
Galantamine Official FDA information, side effects and uses. A B C D E F G H I J K L M N O P Q R S T U V W X Y Z Galantamine hydrobromide is a reversible, competitive acetylcholinesterase inhibitor. Galantamine hydrobromide is known chemically as (4aS,6R,8aS)-4a,5,9,10,11,12-hexahydro-3-methoxy-11-methyl-6H-benzofuro [3a,3,2-ef][2]benzazepin-6-ol hydrobromide. It has an empirical formula of CGalantamine, a tertiary alkaloid, is a competitive and reversible inhibitor of acetylcholinesterase. While the precise mechanism of Galantamine's action is unknown, it is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. If this mechanism is correct, Galantamine's effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that Galantamine alters the course of the underlying dementing process.The ability of Galantamine to produce an overall clinical effect was assessed using a Clinician's Interview Based Impression of Change that required the use of caregiver information, the CIBIC-plus. The CIBIC-plus is not a single instrument and is not a standardized instrument like the ADAS-cog. Clinical trials for investigational drugs have used a variety of CIBIC formats, each different in terms of depth and structure. As such, results from a CIBIC-plus reflect clinical experience from the trial or trials in which it was used and cannot be compared directly with the results of CIBIC-plus evaluations from other clinical trials. The CIBIC-plus used in the trials was a semi-structured instrument based on a comprehensive evaluation at baseline and subsequent time-points of 4 major areas of patient function: general, cognitive, behavioral and activities of daily living.
